Clinical research is an investigation that involves people.
Clinical research can be divided into two types – observation studies and clinical trials.
What is a clinical trial?
Constant research is needed to improve health outcomes. Advances in medical research and health practices would not be possible without clinical trials.
Clinical trials find new or better ways to improve health by trialling them in people in a regulated and safe environment.
Clinical trials can test many things, including:
- testing new treatments;
- combining treatments to see if they're more effective than standard practice;
- testing a new procedure;
- testing medical devices;
- trialling different strategies to reduce symptoms;
- testing components in the blood or tissue to help detect a disease;
- improving patient outcomes with education or communication interventions.
Participating in trials is voluntary. A consent form must be signed before patients can be involved in any trial procedure. After consent is given, you can choose to withdraw consent and stop the trial at any time.
Clinical trials have strict rules and scheduled events the team must follow. The regulations and scheduled events have been designed to make sure participants are safe. Every participant needs to pass a strict eligibility criteria before they can join a trial. The research team then decide whether a patient is eligible for a clinical trial and invite them to participate. If a patient consents to participate, the study coordinator organises their study tasks and appointments.
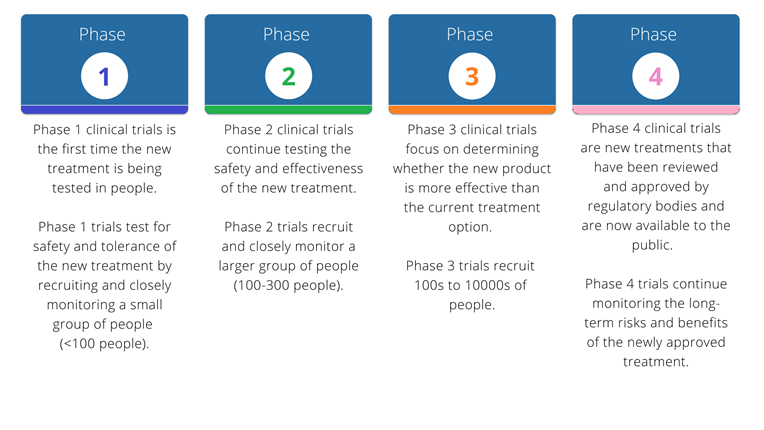
Clinical trials occur in different phases from Phase 1 through to Phase 4 and each phase has a different purpose. It is important to understand what phase the trial is in to decide if being part of the trial is the best option for you. The trial phase is included in the title of the clinical trial. Clinical trials at Grampians Heatlh mainly run in phase 2, 3 or 4.
Why are clinical trials important?
Clinical trials provide evidence that a new intervention is more effective than what is already in place. Clinical trials are essential in raising health care standards and improving patient outcomes. This is how we keep improving health care.
New products or procedures being investigated in a clinical trial for a particular disease are only available to participants in that trial. Therefore, clinical trials can be an opportunity for members of the community to gain access to treatment and services for their disease that may not be otherwise available. Having access to clinical trials gives patients and their doctors more options when treating their disease and symptoms.
Have something to tell us? We welcome all feedback from patients, family members or carers. Tell us more.

